PMC MOCK TEST 5 WITH ANSWERS 2022
PMC MOCK TEST 3 with ANSWERS 2022
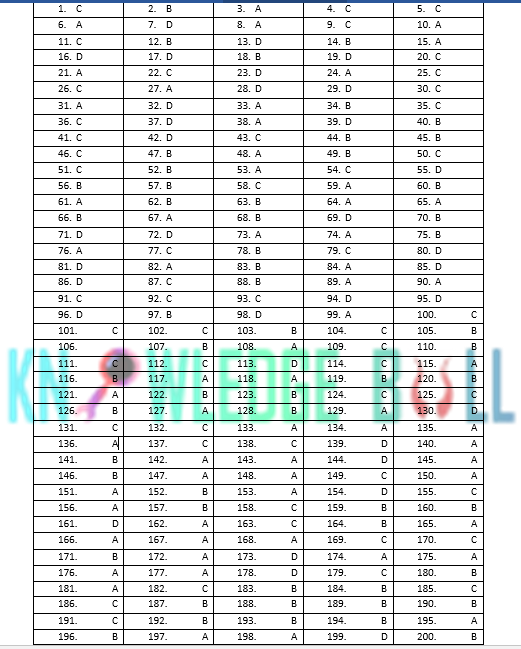
PMC MOCK TEST 4
1 HIV use the RNA polymerase to make the mRNA in host body, The RNA polymerase is of :
a. Virus that is synthesized in the same host
b. Host that is synthesized in the its previous generation
c. Host that is synthesized in the its current generation
d. Virus that is synthesized in the previous host
2. When HIV attack upon its host target cell:
a. Its RNA genome is broken down immediately
b. Its RNA genome is replicated first and then broken down
c. Its RNA genome is converted into viral DNA first and then broken down
d. Its RNA genome immediately causes breakdown of host DNA
3. Another problem for those studying viral origins and evolution is their high rate of mutation, particularly the case in RNA retroviruses like :
a. HIV
b. Bacteriophages
c. Pox viruses
d. HAV
4. A virologist who studies viruses in great detail which of the following would he use?
a. Compound microscope
b. Porcelain filters
c. Electron microscope
d. CT Scan machine
5. During Calvin cycle one molecules of carbohydrate is yield by
a. 2 molecules of CO2
b. 3 molecules of CO2
c. 6 molecules of CO2
d. 7 molecules of CO2
6. Oxidative phosphorylation during respiratory electron transport chain requires:
a. NAD+ as oxidizing agent
b. NADP+ as oxidizing agent
c. Molecular oxygen
d. Light energy
7. ATP synthase is located in the ________ of the mitochondrion :
a. Intermembrane space
b. Outer membrane
c. Matrix
d. Inner membrane
8. The quantitative study of energy relationship in a biological system is called?
a. Bioenergetics
b. Biochemistry
c. Biotechnology
d. Biophysics
9. The R group of an amino acid represents:
a. A hydrogen atom
b. a hydrocarbon
c. an additional atom or group of atoms
d. a methyl group
10. Dietary fibers are
a. Cellulose
b. Glycogen
c. Starch
d. Proteoglycans
11. At least, how many fatty acid molecules does an acyl glycerol contain?
a. 1
b. 2
c. 3
d. 4
12. Which of the following is the example of globular protein?
a. Keratin
b. Enzymes
c. Fibrinogen
d. Collagen
13. The prime function of ATP is to :
a. Act as a catalyst
b. Describe the function of a cell
c. Act as a template for production of protein
d. Act as energy currency
14. All organic biomolecules must contain:
a. Iron and oxygen
b. Carbon and hydrogen
c. Carbon and nitrogen
d. Carbon and Sulphur
15. R-group of glycine consists of:
a. Hydrogen atom
b. Methyl group
c. Amino group
d. Carboxylic acid group
16. Major component of bacterial cell wall is:
a. Teichoic acid
b. Cartenoid
c. Chitin
d. Murein
17. Carbon dioxide passes through plasma membrane of cells by:
a. Active transport
b. Phagocytosis
c. Facilitated diffusion
d. Simple diffusion
18. Glycoproteins and glycolipids are present :
a. Within lipid bilayer of plasma membrane
b. On outer surface of lipid bilayer of plasma membrane
c. On inner surface of lipid bilayer of plasma membrane
d. As bilayer in plasma membrane
19. What is incorrect about plasma membranes?
a. They are involved in maintaining gradients between different compartments
b. They are involved in cell communication
c. They are involved in exchange of materials
d. They initiate nuclear division
20. What is the difference between facilitated transport and passive transport?
a. All facilitated transport is passive
b. All passive transport is facilitated
c. Facilitated transport requires membrane proteins
d. Passive transport is downhill whereas facilitated transport is uphill
21. Which organelle synthesizes proteins for export or repackaging elsewhere?
a. Rough endoplasmic reticulum
b. Smooth endoplasmic reticulum
c. Free ribosomes
d. Golgi complex
22. Lysosomes originate from which cell organelle?
a. Rough endoplasmic reticulum
b. Smooth endoplasmic reticulum
c. Golgi apparatus
d. Cell membrane
23. The components of coordinated actin include which of the following?
a. Stimulus and receptor
b. Coordinator and effectors
c. Response
d. Receptors, neurons & effectors
24. Which of the following neuron has a single long dendron?
a. Sensory neuron
b. Motor neuron
c. Associative neuron
d. Interneuron
25. The blind spot in the eye is so-called due to the absence of which of the following?
a. Rods only
b. Cones only
c. Rods and cones
d. Retina
26. The relay center between various parts of the brain and spinal cord is called?
a. Hypothalamus
b. Thalamus
c. Midbrain
d. Hindbrain
27. Degenerating brain cells can be repaired by using which of the following cells?
a. Embryonic stem cells
b. Schwann cells
c. Neurons
d. Ganglia
28. Which of the following are collectively called the brain stem?
a. Thalamus, hypothalamus and midbrain
b. Cerebellum, pons and medulla oblongata
c. Thalamus, hypothalamus and medulla
d. Medulla oblongata, pons and midbrain
29. Which of the following is true about cnidarians?
a. They are deuterostomic animals
b. They are triploblastic animals
c. They are protostomous animals
d. They are diploblastic animals
30. Which of the following animal phylum is included in series deuterostomia?
a. Platyhelminthes
b. Annelida
c. Chordata
d. Mollusca
31. Which of the following invertebrate phylum consists of the most abundant species on planet Earth:
a. Porifera
b. Cnidaria
c. Arthropoda
d. Mollusca
32. Which among the following lacks the nervous system
a. Flat worms
b. Annelida
c. Cnidaria
d. Sponge
33. Inhibitng enzymatic activity by blocking its active site is:
a. Competitive inhibition
b. Non-competitive inhibition
c. Feedback inhibition
d. Allosteric inhibition
34. All enzymes are composed of:
a. Triglycerides
b. Amino acids
c. Monosaccharides
d. Phospholipids
35. What happens when an enzyme gets denatured by extreme pH?
a. Its amino acids are ionized
b. Its number of amino acids is changed
c. Tertiary structure gets disrupted.
d. It temporarily stops its activity
36. The globular structure of enzymes shows:
a. Primary structure of protein
b. Secondary structure of protein
c. Tertiary structure of protein
d. That it’s an organic catalysis
37. The energy required to start a reaction is called?
a. Startup energy
b. Initial energy
c. Point energy
d. Activation energy
38. Darwin belived that:
a. Natural selection and adaptations are closely related processes
b. Natural selection and special creation are closely related processes
c. Use and disuse of organs and inheritance of acquired characters are closely related processes
d. Evolution and mutation are closely related processes
39. Which of these is vestigial organ of whale?
a. Flipers
b. Fins
c. lungs
d. pelvis and leg bones
40. A group of individual that have the potential to interbreed in nature is:
a. Genus
b. Species
c. Population
d. Community
41. According to Hardy-Weinberg theorem, frequencies of alleles and genotypes in a population’s gene pool remain:
a. unaffected from environmental factors
b. Dynamic unless acted upon by agents other than sexual recombination
c. static unless acted upon by agents other than sexual recombination
d. Constant unless acted upon by agents other than sexual recombination
42. Where lymph capillaries end blindly?
a. Lymph vessels
b. Tissues
c. Abdominal Cavity
d. Thoracic Cavity
43. Which of the following vessels have larger intercellular spaces in their walls?
a. Lymph vessels
b. Blood capillaries
c. Arterioles
d. Venules
44. Finally, lymph vessels deposit the lymph into :
a. Blood capillaries
b. Veins
c. Arterioles
d. Lymph nodes
45. Backflow of lymph is controlled by :
a. Breathing movement
b. Valves
c. Lymph nodes
d. Thoracic lymphatic duct
46. Lacteals within villi of small intestine are the type of :
a. Blood capillaries
b. Arterioles
c. Lymph capillaries
d. Venules
47. Which provide long lasting protection against any infection?
a. Active immunity
b. Passive immunity
c. Skin
d. Phagocytes
48. Which of the following is true about carnivorous plants?
a. They always remain heterotrophic
b. They always remain autotrophic
c. They are autotrophic organisms but sometimes show heterotrophic mode
d. They are heterotrophic organisms but sometimes show autotrophic mode
49. Which of the following is a circular, double stranded DNA molecules present in some bacterial cells?
a. Nucleiod
b. Plasmids
c. Mesosomes
d. Chromosomes
50. Which of the following provide resistance against some antibiotics such as beta lactam?
a. Gram negative cell wall
b. Spores
c. Slime
d. Capsules
51. A very delicate surrounding that may result in death of bacteria if damaged is called :
a. Capsule
b. Flagella
c. Cell membrane
d. Cell wall
52. Protein factories in prokaryotic cell are:
a. Mesosomes
b. Ribosomes
c. Plasmids
d. Nucleotide
53. During sterilization process, in order to control pathogens, we use dry heat which causes
a. Oxidations of bacterial compounds
b. Coagulation of bacterial proteins
c. Separate the bacteria from their media
d. Decrease in bacterial growth
54. The hormone that is released from the testes is?
a. Progesterone
b. Estrogen
c. Testosterone
d. Interstitial cell stimulating hormone
55. Which of the following is haploid cell?
a. Spermatogonia stem cell
b. Spermatogonium
c. Primary spermatocyte
d. Secondary spermatocyte
56. Which of the following is included in male genitalia:
a. Cervix
b. Seminepherous tubules
c. Corpus luteum
d. Graafian follicle
57. The human menstrual cycle generally repeats after how many days?
a. 20 days
b. 28 days
c. 10 days
d. 40 days
58. An egg is fertilized in laboratory and implanted in uterus for development. Which of the following term is related to this procedure:
a. Leutinzatioin
b. Menopause
c. In vitro fertilization
d. Incubation
59. In earthworm, contraction of which muscles shortens the body?
a. Longitudinal
b. Circular
c. Protractor
d. Adductor
60. Tricep muscles are:
a. Flexor muscles
b. Extensor muscles
c. Adductor muscles
d. Abductor muscles
61. Bicep muscles are:
a. Flexor muscles
b. Extensor muscles
c. Adductor muscles
d. Abductor muscles
62. Which of the following muscles are controlled by somatic nervous system:
a. Heart muscles
b. Skeletal muscles
c. Involuntary muscles
d. Smooth muscles
63. When Mendel conducted his experiments, he needed to transfer pollen grains from a male reproductive organ of a female reproductive organ of the same plant What is this process called:
a. Cross pollination
b. Self polination
c. Hybridization
d. Test cross
64. The traits that shows autosomal inheritance have:
a. Equal probabilty in male and female individuals
b. Different probabilty in male and female individuals
c. More probabilty in male than female individuals
d. More probabilty in female than male individuals
65. Crossing over brings:
a. Recombination of genes
b. New trait into species
c. Reduction in number of chromosomes
d. Segregation of genes
66. If a trait passes from father to all his daughter but none of his son, the trait is:
a. X-linked recessive
b. X-linked dominant
c. Autosomal recessive
d. Autosomal dominant
67. Two phenotypically normal individuals have an affected child What can we conclude about the parents?
a. Both parent carried the disease allele
b. Both parents are homozygous for their trait
c. They are not the parents of the child
d. No conclusion can be drawn
68. An Rh- woman is married to Rh+ man whose father was Rh- The risks of Erythroblastosis fetalis baby can be:
a. 25%
b. 50%
c. 75%
d. 100%
PHYSICS
69. A ball is projected with kinetic energy E at 45 degree angle. At highest point its kinetic energy is:
a. 2E
b. 0
c. E
d. E/2
70. In a projectile motion, velocity at maximum height is:
a. usinθ
b. ucosθ
c. utanθ
d. ucotθ
71. The horizontal range is 4√3 times of the maximum height.
Its angle of projection is:
a. 90 degree
b. 45 degree
c. 60 degree
d. 30 degree
72. At what angle of projectile horizontal range is minimum:
a. 30 degree
b. 45 degree
c. 60 degree
d. 90 degree
73. For a projectile at angle (45+θ) and (45-θ), the ratio of horizontal range described by the projetile are:
a. 1.00
b. 0.50
c. 2.00
d. 0.08
74. A car and truck is running with same velocity, a constant and same force applied to both, which will stop first:
a. Car
b. Truck
c. Both at same time
d. Information insufficient
75. A block moves in uniform circular motion because a cord tied to the block is anchored at the center of the circle. What will be the power exerted on the block by the cord?
a. Positive
b. Zero
c. Negative
d. Variable
76. A car is running with velocity 50 m/s and then driver puts brake and car comes to rest. The force applied by car in the opposite direction is 2 N.
What is the power exerted by the engine?
a. -100 watt
b. 0 watt
c. 25 watt
d. 100 watt
77. An engine pumps 400 kg of water through a height of 10 m in 40 s. Find the power of engine if its efficiency is 80%? Take g = 10ms^-2.
a. 1.25 W
b. 125 W
c. 1.25 kW
d. 12.5 kW
78. A block of mass 2 kg is moving with the velocity 5 m/s in the horizontal direction under an influence of force 20 N. How much power is delivered by normal force?
a. -100 W
b. 100 W
c. 0 W
d. 200 W
79. One complete revolution of an object covers _____ angle in degree
a. 180
b. 90
c. 360
d. 270
80. Earth revolution in 24 hours covers _____ angle:
a. 90
b. 60
c. 57.3
d. 360
81. The rotation of earth in 1 day covers ____ radian angular distance approximately:
a. 6.3
b. 7.3
c. 9.3
d. 5.3
82. In multiple revolutions, centre of rotation is______
a. Fixed
b. Not fixed
c. May depend on condition of rotation
d. There is no centre in angular motion
83. Stationary waves are produced by:
a. Reflection
b. Interference
c. Diffraction
d. Rarefaction
84. When two ends of string are struck together simultaneously such that two troughs are traveling towards each other, than at reaching the centre of string ____ is formed:
a. Higher amplitude
b. Lower amplitude
c. Waves die immediately
d. Troughs converted to crest
85. Waves which always need medium to travel are _______ waves:
a. Light
b. Stationary
c. Sound
d. Both sound and stationary
86. Stationary waves cannot be produced in:
a. Water waves
b. Finite ropes
c. Sound waves
d. Light waves
87. The Doppler effect in sound waves is observed because
a. Sound waves are longitudinal
b. Sound waves are transverse
c. the distance between the source of sound and the observer is changing
d. the distance between the source of sound and the observer is fixed
88. A pipe open at both ends resonates at a fundamental frequency f_o. When one end is covered and the pipe is again made to resonate, the fundamental frequency is f_c. Which of the following expressions describes the relationship between these two resonants?
a. f_c = f_o
b. 2 * f_c = f_o
c. f_c = 2* f_o
d. “”” 2 * f_c = 3* f_o”””
89. Pitch is __________ proportional to Frequency
a. Directly
b. Inversely
c. Not related
d. Exponentionally
90. If Cv= 8 J/K mol then calculate the change in heat for dT = 50 K of 2 mole gas?
a. 800J
b. 1631.4 J
c. 1700 J
d. 1800 J
91. If 2 kg water, having specific heat Cv = 4.186 J/(g C°), heated and the change in temperature is 10 degree C, then heat supplied is:
a. 41860J
b. 90000J
c. 83720J
d. 80000J
92. The value of Cp and Cv is same for:
a. He
b. Ar
c. Aluminium
d. Neon
93. Charge inside conductor is:
a. Possible
b. Can be greater than zero
c. Zero
d. Negative always
94. The electric flux through a closed surface depends upon the:
a. Size of the surface
b. Shape of the surface
c. Position of charge enclosed in the surface
d. Magnitude of charge enclosed in the surface
95. Electric potential determines the flow of?
a. Atom
b. Molecule
c. Compound
d. Charge
96. In parallel plate capacitor Capacitance depends on:
a. Charge
b. Electric field
c. Voltage
d. Crosssectional area
97. In the combination of capacitors in series, the capacitors are connected:
a. Parallel
b. Side by side
c. Up and down
d. In circle only
98. The property of material due to which it attracts or repels other objects is:
a. Friction
b. Velocity
c. acceleration
d. Charge
99. The number of field lines passing through a certain element of area is known as:
a. Electric Flux through that area
b. Electric current through that area
c. Voltage through that area
d. Amperes through that area
100. Which one of the following is a disadvantage of a potentiometer over a voltmeter ?
a. It can measure the internal resistance of a cell
b. It can measure the e.m.f. of a cell
c. It is heavy and not portable
d. It can measure accurately very small pd. of the ord of few microvolt
101. The sensitivity of a potentiometer can be increased by________
a. Increasing the e.m.f. of the primary cell
b. Increasing the potential gradient
c. Increasing the length of the potentiometer wire
d. Decreasing the length of the potentiometer wire
102. The terminal potential difference of a battery is greater than its emf when:
a. The internal resistance of a battery is infinite
b. The internal resistance of a battery is zero
c. The battery is charged
d. The battery is discharged
103. The resistance of a conductor at absolute zero ( 0 K) is:
a. Almost zero
b. Almost infinite
c. No prediction at all
d. May increase or decrease
104. The circuit which gives continuously varying potential is called:
a. Complex network
b. Wheatstone bridge
c. Potential divider
d. Galvanometer
105. A current of 16 amperes divides between two branches in parallel of resistances 8 ohms and 12 ohms respectively. The current in each branch is:
a. 6.4 a, 6.9 a
b. 6.4 a, 9.6 a
c. 4.6 a, 6.9 a
d. 4.6 a, 9.6 a
106. If we cut a magnet in half then both magnets are now:
a. Monopole
b. Dipole
c. Quadrupole
d. Magnetism is lost
107. Magnetic flux is defined as:
a. Magnetic field lines passing over a volume
b. Magnetic field lines passing through a surface
c. Current passing through surface
d. current passing through surface
108. If electron and proton projected in a magnetic field simultaneously then __________ will be deflected more:
a. Electron
b. Proton
c. Electron and proton both
d. No effect
109. The mechanical energy of our hand used to push the magnet towards or away from the coil results into:
a. Heat energy
b. Potential energy
c. Electrical energy
d. Mechanical energy
110. In step down transformer, current in secondary coil is ____ than current in primary coil:
a. Equal
b. Less
c. Greater
d. Unchanged
111. Power loss in the actual transformer is due to:
a. Back emf
b. Strong magnetic field
c. Eddy currents
d. Weak magnetic field
112. AC generator cannot generate:
a. AC voltage
b. High AC voltage
c. DC voltage
d. Low AC voltage
113. AC generators are similar to:
a. Transformers
b. Inductors
c. Capacitors
d. Motor
114. Most widely used rectifier is:
a. Half wave rectifier
b. Full wave rectifier
c. Bridge rectifier
d. Loop rectifier
115. Where does the energy lost by fast moving electron goes:
a. Appears as photon
b. Appears as electron – positron pair
c. Appears as its k.e.
d. It vanishes
116. When gamma photon is entered in nucleus it_____:
a. De-excite the nucleus
b. Excite the nucleus
c. Scatter by nucleus
d. No effect on nucleus
117. In annihilation process particles move in:
a. Same direction
b. Opposite direction
c. Perpendicular direction
d. Remain stationary
118. Lasers are produced by:
a. Stimulated Emission
b. Spontaneous Emission
c. Absorption
d. Random Absorption
119. Bright Lines in pattern shows:
a. Absorption
b. Emission
c. Reflection
d. Free Particle
120. C-14 decays by emitting a beta particle. After decay new element becomes:
a. C-13
b. N-14
c. O-15
d. O-16
121. Which radiation will be deflected by magnetic field?
a. ɑ radiation
b. ϒ radiation
c. X-rays
d. Electromagnetic radiation
122. Naturally occuring fusion reaction source is:
a. Moon
b. Sun
c. Earth
d. Satellite
CHEMISTRY
123. In the process of iron rusting, which of the following is limiting reactant?
a. Iron
b. Oxygen
c. Air
d. Water
124. The limiting reactant in a perticular chemical reaction is the one which:
a. Is consumed later in the reaction.
b. Is present in excess in the reaction.
c. Gives least amount of product.
d. Gives large amount of product.
125. The limiting reactant in a particular chemical reaction is the one which:
a. Is consumed later in the reaction
b. Is present in excess in the reaction
c. Gives least amount of product
d. Gives large amount of product
126. Which is the correct demonstration of de-Broglie equation?
a. λ=mv/h
b. λ=h/mv
c. h=λ/mv
d. mv=hλ
127. What is the value of Planck’s constant (h)?
a. 6.62×10^-34 Js
b. 6.02×10^-34 Js
c. 6.62×10^-33 Js
d. 6.34×10^-34 Js
128. Which of the following orbital is closer to nucleus ?
a. 3d
b. 2p
c. 3p
d. 3s
129. Mass of electron _____ times smaller than that of proton.
a. 1837
b. 1839
c. 1836
d. 1834
130. Boyle’s law is:
a. quantitative relation ship between pressure and number of molecues of a fixed amount of a gas at STP
b. quantitative relation ship between pressure and volume of a fixed amount of a gas at contant volume.
c. quantitative relation ship between pressure and volume of a fixed amount of a gas at contant pressure.
d. quantitative relation ship between pressure and volume of a fixed amount of a gas at contant temperature.
131. Standard temperatue in Fahrenheit (℉) is:
a. -273℉
b. 0℉
c. 32℉
d. 114℉
132. The quantitative relationship between absolute temperature (T) and volume (V) of a fixed amount of a gas at constant pressure (P) can be best describe by:
a. VT = K
b. V / P = T
c. V / T = K
d. P = KT
133. Which of the following substances has the largest intermolecular forces of attraction ?
a. H₂O
b. H₂S
c. H₂Se
d. H₂Te
134. Which of the following substances has the largest intermolecular forces of attraction ?
a. H₂O
b. H₂S
c. H₂Se
d. H₂Te
135. Which of the following crystal have highest density ?
a. Iodine
b. Graphite
c. Ice
d. Sodium chloride
136. Polymorphs have same
a. Chemical Properties
b. Melting Points
c. Structures
d. boiling point
137. Which of the following solutions will act as a buffer?
a. HNO₂ and NaNO₂
b. HCl and KCl
c. HNO₃ and NH₄NO₃
d. NaOH and NaCl
138. The pH of a solution can be kept constant by:
a. saturated solution
b. super saturated solution
c. Buffer solution
d. dilute solution
139. Common Ion Effect finds its extensive application in:
a. Quantitative analysis
b. Gas testing
c. Material testing
d. Salt Analysis
140. [Product]/[Reactant] = 2.5 for a reaction at equillibrium, what does it mean?
a. products are more than reactants
b. Products are less
c. Reaction is at equilibrium
d. Reactants are more
141. The number of reactant molecules whose concentrations affect the rate of reaction is called :
a. Rate law
b. Molecularity
c. Order of reaction
d. Rate constant
142. The correct value for the enthalpy of formation of CO is:
a. -110kJ/mol
b. -210kJ/mol
c. +110kJ/mol
d. +210 kJ/mol
143. Born Haber cycle is used to determine:
a. Enthalpy of lattice
b. Enthalpy of formation
c. Enthalpy of neutralization
d. Enthalpy of solution
144. Oxidation state of Cr in K₂Cr₂O₇ is:
a. +3
b. +4
c. +5
d. +6
145. Which of the following metal is highly reactive?
a. Li
b. Zn
c. Ag
d. Au
146. Which type of bond is present between C and O in methanol?
a. Dative bond
b. Polar bond
c. Non-polar bond
d. Ionic bond
147. The covalent radius of H₂ is:
a. 37.7 pm
b. 37.7 nm
c. 37.7 mm
d. 37.7 μm
148. Select among the following that has trigonal pyramidal geometry:
a. NH₃
b. BF₃
c. AlCl₃
d. SO₃
149. The name of a common ore from which aluminium can be extracted is called?
a. Charcoal
b. Haematite
c. Bauxite
d. Cryolite
150. Which of the following doesnot react with water even at red hot temperature.
a. BeO
b. MgO
c. CaO
d. BaO
151. Upon bunring in air, normal oxide is formed by :
a. Li
b. Na
c. K
d. Rb
152. When magnisium nitride (Mg₃N₂) reacts with with water the reaction mixture becomes:
a. Acidic
b. Basic
c. Neutral
d. Amphoteric
153. Atomic numbers of chromium (Cr) is 24. Correct electronic configuration of Cr is:
a. [Ar]3d⁵4s¹
b. [Ar]3d⁵4s²
c. [Ar]3d⁶4s⁰
d. [Ar]3d⁴4s²
154. Which element shows maximum oxidation state?
a. Cu
b. Zn
c. Cr
d. Mn
155. Which of the following fraction of petroleum has lowest boiling point at STP.
a. Kerosine oil
b. Lubricating oil
c. Gasoline
d. Natureal gas
156. Conversion of straight chain hydrocarbons into branched chain is called as_______?
a. Reforming
b. Cracking
c. Isomerization
d. Decomposition
157. Fuels with higher octane number can be produce by______?
a. Cracking
b. Reforming
c. Decomposition
d. Isomerisation
158. Halogenation of alkane in sunlight is the example of:
a. Addition reaction
b. Elimination reaction
c. Substitution reaction
d. Rearrangment reaction
159. Major product of the following reaction is: CH₂=CH-CH₂-CH₃ + HBr →
a. CH₂(Br)-CH₂-CH₂-CH₃
b. CH₃-CH(Br)-CH₂-CH₃
c. CH₂=CH-CH(Br)-CH₃
d. CH₂=CH-CH₂-CH₂(Br)
160. Which of the following process gives benzensulphonic acid from benzene?
a. Ozonolysis
b. Sulphonation
c. Nitration
d. Halogenation
161. Benzene is less reactive than is corresponding alkene due to:
a. More Unsaturation
b. More Saturation
c. Less electronegativity difference between C and H-atom
d. Delocalization of pi electrons
162. What is the structure of benzene?
a. Regular, flat planar hexagon
b. Regular, 3D hexagon
c. Regular, flat planar octagon
d. Regular, planar pentagon
163. Which one of the following is a strong Nucleophile?
a. Cl-
b. Br-
c. OH-
d. HSO4-
164. 2-chlorobutane belongs to:
a. Primary Alkyl halides
b. Secondary Alkyl Halides
c. Tertiary Alkyl Halides
d. Quarternary Alkyl halides
165. If bond energy of C-Cl is 346 kJ/mol. The bond enegy of C-Br should be:
a. 290 KJ/mol
b. 390 kJ/mol
c. 490 KJ/mol
d. 590 KJ/mol
166. Which of the following reactions are given by alcohol and phenol both?
a. Esterification
b. Nitrosation
c. Amide formation
d. Lucas Reaction
167. Which of the following reactions are given by alcohol and phenol both?
a. Esterification
b. Nitrosation
c. Amide formation
d. Lucas Reaction
168. Which of the following reactions are given by alcohol and phenol both?
a. Esterification
b. Nitrosation
c. Amide formation
d. Lucas Reaction
169. Formaldehyde reacts with which one of the following to give primary alcohols
a. Fehling’s reagant
b. Benedict’s Reagant
c. Grignard’s reagant
d. Millon’s reagant
170. Formaldehyde reacts with which one of the following to give primary alcohols:
a. Fehling’s reagant
b. Benedict’s Reagant
c. Grignard’s reagant
d. Millon’s reagant
171. Aldol products upon heating undergoes:
a. Decomposition
b. Dehydration
c. Rearrangement
d. Substitution
172. Which of the following gas is produced during the reaction of carbonates and bicarbonates with carboxylic acid
a. CO₂
b. H₂
c. N₂
d. Ar
173. Acetic acid was first isolated from:
a. Ants
b. Butter
c. Milk
d. Vinegar
174. Which of the following gas is produced during the reaction of carbonates and bicarbonates with carboxylic acid?
a. CO₂
b. H₂
c. N₂
d. Ar
175. The repetition of faces, angles and edges of a crystal, when it is rotated to 360 degree angle is called:
a. Habit of crystal
b. Isomorphism
c. Symmetry
d. Polymorphism
176. Which of the following properties about isomorphs is not True?
a. Isomorphs can make homogeneous mixture together
b. Have different physical properties
c. Have same atoms
d. Have same ratio of atoms
ENGLISH
177. The train was so _________that we could not get into it.
a. crowded
b. deep
c. thick
d. rushed
178. We have a ______ climate so the summers are not very hot.
a. bright
b. fair
c. high
d. mild
179. Do you know how ______ it is from Faisalabad to Islamabad? It’s 470 km.
a. many
b. much
c. far
d. often
180. Choose the present tense.
a. They lived happily.
b. They live happily.
c. They will live happily.
d. They had lived happily.
181. I am going to the U.S.A next year. Which type of tense is it?
a. Present
b. Past
c. Future
d. Interrogative
182. When I was young, I ________________ (use) play cricket a lot.
a. use
b. used
c. used to
d. use to
183. We need to find _______ method to solve this problem.
a. other
b. another
c. others
d. the other
184. The man __________ wallet was stolen, called the police.
a. who
b. whose
c. whom
d. which
185. The market is nearer to them than__________.
a. we
b. ourself
c. us
d. ourselves
186. Choose the correct spelling of the word.
a. Annehilate
b. Anihilate
c. Annihilate
d. Anehilate
187. Choose the correct spelling of the word.
a. Ignomeny
b. Ignominy
c. Ignomminy
d. Ignomny
188. Choose the correct spelling of the word.
a. Exhebition
b. Exhibition
c. Exhabation
d. Exehation
189. Which one is correct?
a. Natasha Ryan returned to her mother home on Thursday night.
b. Natasha Ryan returned to her mother’s home on Thursday night.
c. Natasha Ryan returned to hers mother’s home on Thursday night.
d. Natasha Ryan returned her mother’s home on Thursday night.
190. Which one is correct?
a. A hundreds year ago, boys from this school had fought with the French.
b. A hundred years ago, boys from this school had fought against the French.
c. A hundred years ago, boys from this school was fighting against the French.
d. A hundred years ago, boys from this school were fighting against French.
191. Which one is correct?
a. Norma did not agree on Mr. Steward to have the button unit brought back.
b. Norma did not agree upon Mr. Steward to have the button unit brought back.
c. Norma did not agree with Mr. Steward to have the button unit brought back.
d. Norma did not agreed with Mr. Steward to have the button unit brought back.
192. What are you doing _____ the afternoon?
a. with
b. in
c. during
d. around
193. Are the boys still swimming ____________ the pool?
a. into
b. in
c. on
d. at
194. I’ll show you a picture ____________ the palace.
a. off
b. of
c. in
d. at
LOGICAL REASONING
195. I. Two fighter jets from the air force malfunctioned in the mid-air after taking off from the air force base. II. One of the flying officers ejected whereas the other one died on spot.
a. Statement I is the cause and statement II is its effect.
b. Statement II is the cause and statement I is its effect.
c. Both statements I and II are independent causes
d. Both statements I and II are the effects of independent cause.
196. Statement : It is reported that though Vitamin E present in fresh fruits and vegetables is beneficial for human body, but capsule of Vitamin E does not have the same effect on human body.
Courses of Action : I. The sale of capsule Vitamin E should be banned. II. People should be encouraged to take fresh fruits and vegetables to meet the body’s requirement of Vitamin E.
a. Only I follows
b. Only II follows
c. Only III follows
d. Both I and II follow
197. “Pick the word which is always associated with “”books””.”
a. Pages
b. Learning
c. Pictures
d. Eraser
198. Pick the word which is always associated with: Electrical Energy.
a. Light
b. Cars
c. Power
d. Water
199. An autograph can not exist without?
a. Singer
b. Actor
c. Player
d. Pen
200. l. The car manufacturing companies have recently increased the prices of mid-sized cars. ll. The Government recently increased the duty on mid-sized cars.
a. Statement I is the cause and statement II is its effect.
b. Statement II is the cause and statement I is its effect.
c. Both the statements I and II are independent causes.
d. Both the statements I and II are effects of some common cause
Was this helpful?
4 / 0