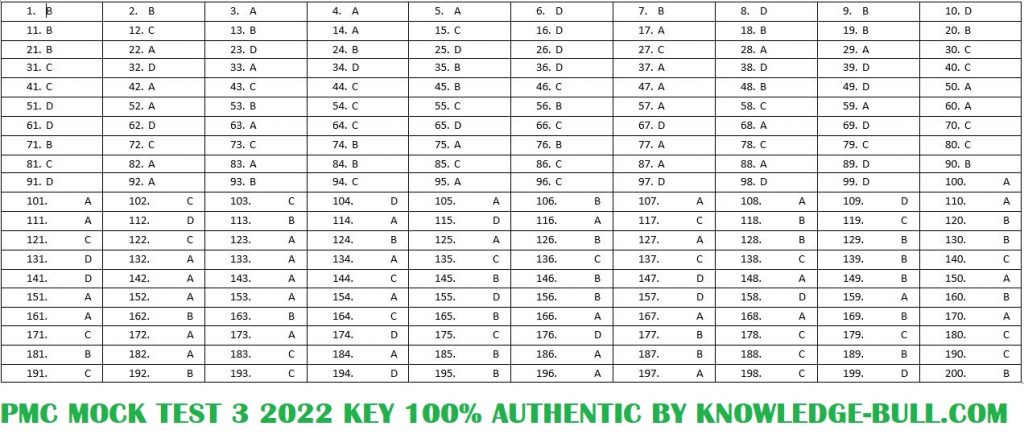
PMC MOCK TEST 5 WITH ANSWERS 2022
PMC MOCK TEST 4 WITH ANSWERS 2022
- A virus has its of genome which is enclosed by a protein coat, called:
a. Spore
b. Capsid
c. Envelop
d. Shell
- Viruses are able to recognize the host surface by means of their:
a. Capsid
b. Spikes
c. DNA
d. RNA - Reverse transcriptase of HIV is used in:
a. Conversion of viral RNA into viral DNA
b. Conversion of viral RNA into host DNA
c. Conversion of viral DNA into viral RNA
d. Conversion of host DNA into viral DNA - Human immunodeficiency virus is:
a. An RNA enveloped virus
b. A DNA enveloped virus
c. An RNA non-enveloped virus
d. An DNA non-enveloped virus - In aerobic respiration, chemiosmosis occurs during :
a. Electron transport chain
b. Photo phosphorylation
c. Glycolysis
d. Kreb’s cycle - One ATP molecule contains 1% of total energy of glucose, In anaerobic reactions how much energy of glucose is released as ATP :
a. 1% b. 2% c. 36% d. 38%
- Which of the following is the final electron acceptor in respiratory electron transport chain?
a. NADP+
b. Molecular oxygen
c. PS-I
d. PS-II - Neil investigation on photosynthesis was carried on :
a. Cyanobacteria
b. Angiosperms
c. Algae
d. Bacteria - Which of the following is milk sugar?
a. Sucrose
b. Lactose
c. Maltose
d. Starch - When two amino acids such as glycine and alanine are variously arranged to make dipeptides. How many different dipeptide combinations can be produced?
a. 1
b. 2
c. 3
d. 4 - Which of the following bonds maintain the primary structure of a protein
a. Hydrogen bonds
b. Peptide bonds
c. Disulphide bonds
d. Hydrophobic interaction
- Maximum how many carbon atoms does an oligosaccharide contain?
a. 3
b. 7
c. 70
d. Many thousands - Cholesterol is not present in______
a. milk
b. egg white
c. egg yolk
d. fish - Hydrogen bonding produces a regular coiled arrangement in proteins called
a. α-helix
b. β-helix
c. Tertiary conformation
d. Quaternary structure - Which of the following is correct about globular proteins?
a. Insoluble in water
b. Elastic in nature
c. Can be crystalized
d. their final structure is secondary - Which cell organelle is incorrectly matched with its function?
a. Chloroplast – carbon fixation
b. Mitochondria – ATP synthesis
c. Cell membrane – cell recognition
d. Golgi apparatus – intracellular digestion - Which interactions are responsible for the formation of lipid bilayer in the watery environment of the body?
a. Hydrophobic interactions between fatty acid tails b. Hydrophobic interactions between phosphate heads c. Hydrophilic interactions between fatty acid tails d. Hydrogen bonding between fatty acid tails
- Which of the following is responsible for giving firmness and box-like shape to plant cells?
a. Cell wall
b. Cell wall and vacuole
c. Nucleus and vacuole
d. Cell membrane - Golgi complex was discovered by which scientist?
a. Robert Brown
b. Camillo Golgi
c. De Duve
d. Robert Hooke - Which of these is the right combination of molecules which make up the secondary cell wall in plant cells?
a. Cellulose, lignin and xylem
b. Cellulose, hemicellulose and lignin
c. Cellulose only
d. Cellulose, hemicellulose and pectin - Which of the following is the system of elongated sac like structures (cisternae) or tubes linking the cell surface membrane and the nuclear envelope?
a. Microtubules
b. Endoplasmic reticulum
c. Golgi complex
d. Microfilaments - Which one of the following can easily pass through plasma membrane
a. Fat soluble substances b. Proteins c. Highly charged materials d. Polysaccharides
- Endemic goiter relates to which altered function?
a. Increased Pancreas function
b. Increased Thyroid function
c. Decreased Pancreas function
d. Decreased Thyroid function - How many hormones does the Islets of Langerhans secrete?
a. 1
b. 2
c. 3
d. 4 - The anterior part of cerebral hemisphere located in the cerebrum is classified as?
a. Ganglion bulbs
b. Axon bulbs
c. Dendrite bulbs
d. Olfactory bulbs - Grave’s disease is produced due to which of the following?
a. Under Secretion of thyroxine
b. Under Secretion of corticosteroids
c. Overproduction of corticosteroids
d. Overproduction of thyroxine - Insufficient secretion of thyroxine in adults causes which of the following disorders?
a. Myxedema
b. Cretinism
c. Goiter
d. Addison’s disease - Which of the following is second largest part of the brain?
a. Cerebellum
b. Cerebrum c. Hypothalamus d. Thalamus
- Which of the following is true about cnidarians?
a. They are deuterostome animals
b. They are triploblastic animals
c. They are protostomes animals
d. They are diploblastic animals - Which of the following animal phylum is included in series deuterostome?
a. Platyhelminthes
b. Annelida
c. Chordata
d. Mollusca - Which of the following invertebrate phylum consists of the most abundant species on planet Earth:
a. Porifera
b. Cnidaria
c. Arthropoda
d. Mollusca - Which among the following lacks the nervous system
a. Flat worms
b. Annelida
c. Cnidaria
d. Sponge - The enzyme-substrate complex is formed in which part of the enzyme molecule?
a. Binding site
b. Allosteric site
c. Catalytic site
d. On all over the surface of enzyme - In non-competitive inhibition, the extent of inhibition depends only on?
a. Concentration of enzyme
b. Concentration of substrate
c. Concentration of ES complex
d. Concentration of inhibitor
- When the salivary amylase is reached in stomach cavity:
a. It becomes an apoenzyme
b. It is denatured
c. It participates in protein digestion
d. It remains unaffected - Which of the following may
attach on active site of an enzyme?
a. Products
b. Coenzyme
c. Activator
d. Inhibitor - Which of the following part of enzyme recognize the substrate?
a. Binding site
b. Active site
c. Catalytic site
d. Overall structure of an enzyme - Which of the following shows significant effect of Genetic drift ?
a. Animal population
b. Plant population
c. Large population
d. Small population - Which of the following was proposed by Lamarck in his theory?
a. Overproduction
b. struggle for existence
c. Natural selection
d. Use and disuse of organs - Most likely, people who perform tough manual work can develop
a. thick subcutaneous fat in their palms b. greater quantity of melanin all over the body c. Strong calf muscles d. certain acquired characters
- According to Darwin, the most
critical factor for evolution is:
a. Migration
b. Mutation
c. Natural selection
d. Genetic drift - Lungs are spongy due to the presence of million of?
a. Alveoli
b. Parabronchi
c. Air sacs
d. Bronchioles - Functional units of lungs are called which of the following?
a. Alveoli
b. Parabronchi
c. Air sacs
d. Bronchioles - Which of the following fish drink large amount of seawater and excrete concentrated urine resulting in maximum salt excretion and minimal water loss?
a. Hagfish
b. Freshwater fish
c. Bony fish
d. Cartilaginous fishes - Which respiration is directly involved in the production of energy, necessary for all living activities?
a. Organismic
b. Cellular
c. Breathing
d. External respiration
- The mechanism of regulation, generally between organism and its environment, of solutes and the gain and the loss of water is
called?
a. Hemostasis
b. Homeostasis
c. Osmoregulation
d. Thermoregulation - Iliac vein from the lower limb’s unit to form:
a. Vena cava
b. Renal vein
c. Hepatic portal vein
d. Cardiac vein - Plasma proteins synthesized in:
a. Gall bladder
b. Liver
c. Spleen
d. Skin - A bacterial cell envelop does not include :
a. Slime
b. Campsule
c. Cell wall
d. Cell membrane - A periplasmic space is:
a. The space between outer membrane
and peptidoglycan layer of cell wall
b. The space between cell membrane
and cell wall
c. The space within the cell
d. The space between phospholipid
bilayer - Which of the following bacteria
are also called recyclers of nature?
a. Pathogenic bacteria
b. Parasitic bacteria c. Photosynthetic bacteria d. Saprotrophic bacteria
- A chemical which is applied on non living surface in order to control bacteria is called:
a. Disinfectants
b. Antiseptic
c. Vaccines
d. Antibiotics - Which of the following is true about staphylococcus?
a. It is chain of spherical bacteria
b. It is bunch of spherical bacteria
c. It is square of spherical bacteria
d. It is cube of spherical bacteria - End of menstrual cycle in old age is called?
a. Menarche
b. Abortion
c. Menopause
d. Telophase - The union of meiotically produced specialized sex cells from each parents produce?
a. Corpus luteum
b. Embryo
c. Zygote
d. Neuroglial cells - Which of the following is the number of chromosomes in human zygote?
a. n
b. 2n
c. 3n
d. 4n - In reproduction, semen refers to which of the following?
a. Fluid and sperms
b. Blood cells and plasma c. Blood and sperms d. Egg and sperms
- Which of the following part of sperm cell penetrates into the ovum during fertilization?
a. Cytoplasm
b. Nucleus
c. Acrosome
d. Mitochondria - Hip joint and shoulder joint are the examples of:
a. Ball and Socket Joint
b. Hinge Joint
c. Fibrous joint
d. Cartilaginous joints - Cardiac tissue is made up of :
a. Cardiac muscle
b. Smooth muscles
c. Skeletal muscles
d. Gut muscles - The composition of skeletal muscle is:
a. Myosin only
b. Actin only
c. Actin, myosin and troponin
d. Actin, myosin and tropomyosin - The skeletal system consists of:
a. All bones in body
b. All muscles and tendons
c. All body organs both soft and hard
tissues
d. All the bones in the body and tissues
that connect them - What is the ratio of homozygous to heterozygous individuals in F2 generation of monohybrid cross
a. 1 ratio 1
b. 1 ratio 2 c. 1 ratio 3 d. 3 ratio 1
- The genetic information for a specific character is determined by
a. Genome
b. Transposon
c. Gene
d. Trait - Blood group AB negative can receive:
a. Blood group A posative
b. Blood group B posative
c. Blood group O posative
d. Any of the negative blood group - Blood group O negative can receive:
a. Blood group A positive
b. Blood group B positive
c. Blood group O negative
d. Any of the positive blood group - Which of the following is the wildtype phenotype of eye color in Drosophila
a. Black eyes
b. White eyes
c. Light red eyes
d. Bright red eyes - Which of the following disease has greater incidence in human male than female?
a. Haemophilia
b. Cystic fibrosis
c. Thalassemia
d. Cretinism - If the bird catches its prey from a height which was initially at rest then momentum will conserve for: a. Bird b. Prey c. Either bird and prey d. Both bird and prey
- A wagon of mass 1000 kg moves 50 km/h on smooth rails. Later, a mass of 250 kg is placed in the wagon. What is the velocity
with which it moves?
a. 20 km/h
b. 50 km/h
c. 40 km/h
d. 25 km/h - A force of 50 dynes is acted on a body of mass 5 g which is at rest for an interval of 3 seconds, then impulse is
a. 0.00015 N.s
b. 0.0015 N.s
c. 0.15 N.s
d. 1.5 N.s - The total momentum of a flock of identical birds could be zero only if the birds are:
a. Taking off from the ground
b. Flying in the same direction
c. Flying in different directions
d. Very tired and coming down to rest - Which of the following quantities has no effect on simple projectile motion?
a. Velocity
b. Force
c. Mass
d. Acceleration - If a particle launched with 5m/s at angle of 45 degree, then time of flight is:
a. 1.44 s b. 0.72 s c. 0.6 s d. 0.5 s
- Kinetic energy of an oscillating simple pendulum at the equilibrium position is:
a. Maximum
b. Minimum
c. Zero
d. Information insufficient - A car and a train both have same kinetic energy. Which one has greater momentum?
a. Car
b. Train
c. Both have same
d. No change - Unit of power in British engineering system is:
a. Horse power
b. Watt
c. Kilowatt
d. Joule - A 10kW motor pumps out water from a well 10 meter deep. What is the quantity of water pumped out per second?
a. 9.2 kg
b. 9.5 kg
c. 10.2 kg
d. 10.8 kg - The acceleration produced by the centripetal force is always:
a. Directed parallel to the center of the circle
b. Directed perpendicular to the center of the circle
c. Directed towards the center of the circle. d. Directed away from the center of the circle
- The earth rotates 360 ∘ about its axis in about 24 hours. By how many degrees will it rotate in 2 hours?
a. 90
b. 60
c. 30
d. 15 - The direction associated with angular displacement is given by:
a. Left-hand rule
b. Head to tail rule
c. Right-hand rule
d. Fleming left hand rule - Centripetal force is provided by________ in a frictional road around a circular path:
a. Friction force
b. Normal force
c. Gravitational force
d. Pseudo forces - Speed of sound does not depend on:
a. Pressure
b. Density
c. Adiabatic index
d. Amplitude - The speed of sound cannot be greater than:
a. Speed of shock wave
b. Speed of light
c. Speed of water wave
d. Speed of sound in vacuum - The sound waves have no:
a. Frequency of the wave
b. Energy c. Nodes d. Intensity
- Sound waves are considered as____ waves:
a. Compressional
b. Transverse
c. Longitudinal
d. Shock - Stationary waves are produced by:
a. Reflection
b. Interference
c. Diffraction
d. Rarefaction - When two ends of string are struck together simultaneously such that two troughs are traveling towards each other, than at reaching the center of string __
is formed:
a. Higher amplitude
b. Lower amplitude
c. Waves die immediately
d. Troughs converted to crest - Waves which always need medium to travel are _ waves:
a. Light
b. Stationary
c. Sound
d. Both sound and stationary - For a monatomic ideal gas of 6 mole Cp /Cv:
a. 1.4
b. 1.67
c. 5
d. 3 - Cp for monatomic gas in terms of R is:
a. 3/5 R
b. 5/3 R
c. 5/4 R
d. 5/2 R
- Cp = 49 J/K.mole and Cv= 35 J/K,mol then what type of gas is this?
a. Diatomic
b. Monatomic
c. Triatomic
d. Nonlinear triatomic - Which of the following forces are only attractive?
a. Electrostatic forces
b. Gravitational forces
c. Magnetic forces
d. Forces between same poles of magnet - Which of the following forces are non conservative?
a. Electric force
b. Gravitational force
c. Frictional forces
d. Elastic spring force - Parallel combination of two capacitor C1 and C2 is:
a. C1+ C2
b. (C1)(C2)
c. (C1C2)/(C1+C2)
d. C1/C2 - Charge inside conductor is:
a. Possible
b. Can be greater than zero
c. Zero
d. Negative always - The electric flux through a closed surface depends upon the:
a. Size of the surface
b. Shape of the surface
c. Position of charge enclosed in the d. Magnitude of charge enclosed in the surface
- Electric potential determines the flow of?
a. Atom
b. Molecule
c. Compound
d. Charge - In parallel plate capacitor Capacitance depends on:
a. Charge
b. Electric field
c. Voltage
d. Cross-sectional area - Ohm’s law is valid at
_ temperatures
a. Constant
b. Varying
c. Low
d. High
101. In order to achieve high accuracy, the slide wire of a potentiometer should be
a. As long as possible
b. As short as possible
c. Neither too small not too large
d. Very thick
- __ is a source of electrical energy having fixed polarity and terminals
a. Motor
b. Metals
c. Battery
d. Generator - Correct form of ohm’s law
a. i = vr
b. v = i/r c. v = ir d. r= iv
- Ohm’s law is valid when the temperature of the conductor is :
a. Very low
b. Very high
c. Varying
d. Constant - The specific resistance of a conductor increases with:
a. Increase in temperature
b. Increase in cross-sectional area
c. Decrease in length
d. Decrease in cross-sectional area - When two magnets come loser such that same poles face each other than field lines:
a. Intersect
b. Repel
c. Come closer
d. Nothing will happen - When two positive charge particles moves towards each other in opposite direction:
a. Repulsion occurs
b. Attraction occurs
c. Nothing will happen
d. Charge will be neutralized - When two positive and negative particles are moving opposite to each other in uniform magnetic field:
a. Deflection occurs
b. Attraction occurs
c. Repulsion occurs
d. Nothing will happen - The induced emf will oppose the flux producing it is according to:
a. Gravitational law
b. Coulomb law
c. Newton’s law
d. Lenze’s law
- The Lenz’s law is used to find out the direction of
a. Current induced in circuit
b. Potential energy
c. Magnetic field
d. Force - Transformer works on principle of :
a. Electromagnetic induction
b. Coulombs law
c. Gauss’s law
d. Ampere’s law - Transformer can convert
a. Low DC voltage
b. High DC voltage
c. Battery voltage
d. AC voltage
- Eddy currents flow in:
a. Open loops
b. Closed loops
c. Straight lines
d. Vertical loops - Ripple factor of half wave rectifier is:
a. 1.21
b. 0.8
c. 0.6
d. 0.4 - Planck constant is named after:
a. Einstein
b. Newton
c. Maxwell
d. Max planck
- Photocell is based on:
a. Photoelectric effect
b. Compton effect
c. Photoluminescence
d. Pair production - A beam of electrons can be:
a. Reflected
b. Refracted
c. Reflected and refracted
d. Polarized - Spectral lines is like a __ of absorbed or emission energy in a spectrum:
a. Charged Pattern
b. Fingerprint Pattern
c. Discharged Pattern
d. Scattered Pattern - The black body always _ radiations:
a. Emit
b. Absorb
c. Both Emit And Absorb
d. Reflects - How many up quarks in proton:
a. 1
b. 2
c. 3
d. 4 - Which particle has no charge:
a. Electron
b. Proton
c. Neutron
d. Positron - The electrons are accelerated in microscopes to produce:
a. X rays
b. Beta rays c. High resolution image d. Gamma ray
- The number of formula units in 10.0 g of CaCO3 are:
a. 6.022×1022
b. 6.022×1023
c. 3.011×1022
d. 3.011×1023 - In a particular chemical reaction the actual yield was 70gm, while that of its theoretical yield is 100 gm. The percentage yield of the said reaction is:
a. 100%
b. 70%
c. 40%
d. 20% - In the process of iron rusting, which of the following is limiting reactant?
a. Iron
b. Oxygen
c. Air
d. Water - Which of the following doesn’t have complete octet?
a. O 2−
b. N5+
c. Ca2+
d. K+ - The value of Azimuthal quantum number (l) depends upon:
a. n
b. m
c. s
d. p
- In the discharge tube positive rays move in which direction?
a. Towards anode
b. Towards cathode
c. Move randomly
d. Move towards vacuum in the tube - What is the shape of s-orbital?
a. Lobed
b. Spherical
c. Dumbell
d. Sausage - According to kinetic theory, which of the following statements is/are correct? a. Gases consist of particles (atoms or molecules)in continuous random motion. b. Collisions between particles are
elastic. c. The volume occupied by
particles is negligibly small d. Attractive forces between particles
are significant at room temperature
and pressure.
a. a and b
b. a, b and c
c. b and c
d. only d - 1 atm is equal to the following EXCEPT:
a. 760 mm of Hg
b. 76 cm of Hg
c. 760 torr
d. 1.47 psi - The temperature at which volume of all the ideal gases becomes zero is:
a. 0 K
b. 0 C0
c. 0 F0
d. 32 F0 - Which of the following substances has the largest intermolecular forces of attraction ?
a. H2O
b. H2S
c. H2Se
d. H2Te - Which of the following substances has the largest intermolecular forces of attraction ?
a. H2O
b. H2S
c. H2Se
d. H2Te - Which of the following solids shows the phenomenon of Isomorphism ?
a. Molecular solids
b. Covalent solids
c. Ionic solids
d. Metallic solids - Who proposed valence bond theory for metals?
a. Loren
b. Drudge
c. L.Pauling
d. Maxwell - Ksp (Solubility product)
depends upon:
a. pressure
b. Concentration
c. Temperature
d. Volume - Nitrogen and Hydrogen present in Equilibrium Mixture in Haber Process remain in
a. Solid state
b. Liquid state c. Gaseous state d. Mixture of solid and gas
- Upon passing Hydrogen chloride gas through a brine solution, which of the following is observed?
a. Concentration of NaCl(aq) decreases
b. Crystalization of NaCl takes place.
c. Concentraion of Na+ ion increases
d. concentrtion of Cl− ion decreases - At ordinary temperature only few molecule possess energy equal to
a. Kinetic Energy
b. Potential Energy
c. Activation energy
d. Ionization Energy - The slowest reaction among these is:
a. Neutralization
b. Double Decomposition Reactions
c. Ionic Reactions
d. Hydrogenation Reaction - Born Haber cycle is used to determine:
a. Enthalpy of lattice
b. Enthalpy of formation
c. Enthalpy of neutralization
d. Enthalpy of solution - Which of the following law is an application of first law of thermodynamics?
a. Hess’s law
b. Dalton’s law
c. Moseley’s Law
d. Avogadro’s law - Cu,Au and Ag are least reactive, because they
have______?
a. Negative reduction potential
b. Negative oxidation potential
c. Positive reduction potential
d. Negative electrode potential
- The element has highest reduction potential in electrochemical series is:
a. Zinc
b. Fluorine
c. Oxygen
d. Lithium - The requirement for bond formation in HF can be met by
a. 2 orbitals & 1 electron
b. 2 orbital & 2 electron
c. 1 orbital & 1 electron
d. 1 orbital & 2 electron - Covalent radius is defined as half of the single bonded length between _ atoms covalently bonded in a molecule.
a. Two Different
b. Two Simultaneous
c. Any Two
d. Two Similar - When repulsive force tend to push the atoms apart and the potential energy of the system is _.
a. No Effect
b. Decrease
c. Increase
d. Change in alternation - The Halide of aluminum is______?
a. Electron deficient
b. Lewis acid
c. High melting point
d. High boiling point
- Among alkali metals which of the following property is high for Li?
a. Hydration energy
b. Ionic radius
c. Electro positivity
d. Atomic size - Among alkaline earth metals the one which shows the least chemical reactivity is:
a. Be
b. Ca
c. Mg
d. Ba - Alkali metal oxides dissolve in water to give:
a. Alkaline solution
b. Neutral solution
c. Acidic solution
d. Buffer solution - Electrons flow from———-
a. Anode to cathode
b. Cathode to anode
c. Don’t flow
d. In either directions - A galvanic cell is setup
a. When a less reactive metal contacts a more reactive metal via an electrolyte
b. When two reactive metals contact via an electrolyte
c. When two reactive metals are placed in any solution
d. When two metals come in direct contact - The breaking of large molecules by heating at high temperature and pressure is called:
a. Reforming
b. Steam cracking c. Catalytic cracking d. Thermal cracking
- Which of the following has anti knocking properties?
a. (CH3CH2)4Sn
b. (CH3CH2)4Pb
c. (CH3CH2)4Ge
d. (CH3CH2)4Si - Which of the following fraction of petroleum has lowest boiling point at STP.
a. Kerosine oil
b. Lubricating oil
c. Gasoline
d. Natureal gas - Benzene is less reactive than is corresponding alkene due to:
a. More Unsaturation
b. More Saturation
c. Less electronegativity difference between C and H-atom
d. Delocalization of pi electrons - What is the structure of benzene?
a. Regular, flat planar hexagon
b. Regular, 3D hexagon
c. Regular, flat planar octagon
d. Regular, planar pentagon - The less reactivity of benzene is due to:
a. Sigma bond
b. Delocalization of pi electrons
c. Less electronegativity of C atom
d. Hexagonal structure - What is the catalyst in Friedel-Crafts Alkylation?
a. AlCl3
b. Al2S3
c. AlH3
d. Al2O3
- In termination step two free radicals combine to form:
a. Another free radical
b. A molecule
c. A molecular ion
d. A cation - The correct name of CH2Cl2 is:
a. Methyl chloride
b. Methylene chloride
c. Chloroform
d. Carbonyl chloride - Which one of the following is
a strong Nucleophile?
a. Cl-
b. Br-
c. OH-
d. HSO4-
- 2-chlorobutane belongs to:
a. Primary Alkyl halides
b. Secondary Alkyl Halides
c. Tertiary Alkyl Halides
d. Quaternary Alkyl halides - Which of the following reactions are given by alcohol and phenol both?
a. Esterification
b. Nitrosation
c. Amide formation
d. Lucas Reaction - Which of the following reactions are given by alcohol and phenol both?
a. Esterification
b. Nitrosation
c. Amide formation
d. Lucas Reaction - Which of the following reactions are given by alcohol and phenol both?
a. Esterification
b. Nitrosation
c. Amide formation
d. Lucas Reaction - Idoform test is used to check the presence of:
a. Amines
b. Carbonyl compounds
c. Carboxylic acid
d. Alkyl halides - Acetone is less reactive than acetaldehyde due to:
a. More Steric hindrance offered by its methyl groups.
b. Electron withdrawing nature of its methyl groups.
c. Its Carbonyl carbon is sp2 hybridized.
d. Oxygen of carbonyl group is more electronegative than carbon atom. - Formaldehyde reacts with which one of the following to give primary alcohols
a. Fehling’s reagant
b. Benedict’s Reagant
c. Grignard’s reagant
d. Millon’s reagant - Complete the following reaction: CH3COOC2H5 + H2O —>.
a. Both HOC2H5 and CH3CO2H
b. Both HOCH3 and CH3CO2H
c. HOC2H5 Only
d. CH3CO2H Only - The aliphatic monocarboxylic acid is obtained from:
a. fats and oils
b. proteins and oils c. fats and proteins d. fats only
- Acetic acid was first isolated from:
a. Ants
b. Butter
c. Milk
d. Vinegar - A solid compound exists in more than one crystalline form, this phenomenon is called:
a. Allotropy
b. Isomorphism
c. Polymorphism
d. Isomerism - Which of the following is not true about Polymorphs?
a. Have same chemical properties
b. Interconvertible
c. Have same formula unit
d. Have different formula unit
ENGLISH - Ali sent his mother a __ of flowers for her birthday.
a. bar
b. bouquet
c. pack
d. packet - Can you please __ your name at the bottom of the contract?
a. cross
b. answer
c. sign
d. lay - The __ climbed up the tree and hid inside the branches.
a. deer b. rabbit c. squirrel d. tortoise
- We will go to Dubai. Which tense is used here?
a. Present
b. Past
c. Future
d. Past Perfect - Which one is future tense?
a. They are going to the market.
b. I will play football.
c. We watched a movie yesterday.
d. He has done his work. - Which one is correct?
a. I will go to the market.
b. I will gone to the market.
c. I will went to market.
d. I will have go to market. - The market is nearer to them than__________. a. we
b. ourself
c. us
d. ourselves - Find the correctly spelt word
a. Sovereign
b. Soveregn
c. Soverean
d. Soverein - Choose the word wrongly spelt.
a. Semester
b. Senesent
c. Sensory
d. Salacious - I _ to learn a lot of new words within one month
a. am going
b. will
c. about
d. can be
- I woke up five o’clock __ the morning.
a. for… in
b. at.. in
c. in… at
d. at… for - The weather is pleasant here__ the spring.
a. above
b. around
c. in
d. on - Choose the correct spelling of the word.
a. Exhebition
b. Exhibition
c. Exhabation
d. Exehation - Choose the correct spelling of the word.
a. Brucracy
b. Beaurocricy
c. Bureaucracy
d. Bueraucracy - Choose the correct spelling of the word
a. Bilding
b. Buillding
c. Building
d. Bulding - Which one is correct?
a. Whatever my father wanted me see was on top of the highest point of my farm.
b. Whatever my father wanted me to see was on top of the highest point of my farm. c. Whatever my father want me to see was on top of the highest point of my farm. d. Whatever my father wanted me to saw was on top of the highest point of my farm.
- Which one is correct?
a. The book that we needed to finish
ours research was missing from the library.
b. The book that we need to finish our research was missing from the library.
c. The book that we needed to finish our research was missing from the library.
d. The book that we needs to finish our
research was missing from the library. - Which one is correct?
a. I set out a breakfast of yogurt and cereal on the kitchen table to my family.
b. I set out a breakfast of yogurt and cereal on the kitchen table from my family.
c. I set up a breakfast of yogurt and cereal on the kitchen table for my family.
d. I set out a breakfast of yogurt and cereal on the kitchen table for my family. - I. There has been opposition by the protestors over the government’s decision of removal of subsidy on fertilizers. II. The government has decided to remove
subsidy on some of the items to
bring reform to the economy.
a. Statement I is the cause and statement II is its effect.
b. Statement II is the cause and statement I is its effect. c. Both statements I and II are independent causes d. Both statements I and II are the effects of independent cause.
- Statement: There was a spurt in criminal activities in the city during the recent festival season.
Courses of Action: I. The police should immediately investigate into the causes of this increase. II. In the future the police should take adequate precautions to avoid the recurrence of such situation during festivals. III. The known criminals should be arrested before any such season.
a. Only I and II follow.
b. Only II follows
c. All I, II and III follow.
d. Either I or II follows - “Pick the word which is always associated with “”books””.”
a. Pages
b. Learning
c. Pictures
d. Eraser - Pick the word which is always associated with: Electrical Energy.
a. Light
b. Cars
c. Power
d. Water - Danial has been visiting friends in his flat for the past two weeks. He is leaving tomorrow
morning and his flight is very early. Most of his friends live fairly close to the airport. Usman lives ten miles away. Farhan lives five
miles away, Numan, seven miles. Ali is farther away than Farhan, but closer than Numan. Approximately how far away from the airport is Ali? a. Nine miles b. Seven miles c. Eight miles d. Six miles
- A boy walks 30 meters in the north direction, after that he takes a right turn and walks 40 meters. After that, he takes another right turn and walks 40 meters more, and finally, he took a right turn and stops after walking 40 meters. Find the distance of The boy from the initial position.
a. 5 m
b. 10 m
c. 15 m
d. 20 m
Was this helpful?
6 / 0